On this page
Publications
Selected publications:
- B.G. Abbott, J.A. Case, S. Dorbala, A.J. Einstein, J.R. Galt, R. Pagnanelli, R.P. Bullock-Palmer, P. Soman R.G. Wells, “Contemporary Cardiac SPECT Imaging – Innovations and Best Practices: An Information Statement from the American Society of Nuclear Cardiology”. J Nucl Cardiol, 2018; 25(5):1847-1860.
- S. Dorbala, K. Ananthasubramaniam, I.S. Armstrong, P. Chareonthaitawee, E.G. DePuey, A.J. Einstein, R.J. Gropler, T.A. Holly, J.J. Mahmarian, M. Park, D.M. Polk, R. Russell III, P.J. Slomka, R.C. Thompson, R.G. Wells, “Single Photon Emission Computed Tomography (SPECT)Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation”. J Nucl Cardiol. 2018; 25(5):1784-1846.
- S.G. Cuddy-Walsh, R.G. Wells, “Patient-specific estimation of spatially-variant image noise for pinhole cardiac SPECT camera.” Med Phys 2018 45:2033-2047.
- R.G. Wells, M. Trottier, M. Premaratne, K. Vanderwerf, T.D. Ruddy, “Single CT for attenuation correction of rest/stress cardiac SPECT perfusion imaging.” J Nucl Cardiol. 2018; 25:616-624.
- R.G. Wells, B. Marvin, M. Poirier, J.M. Renaud, R.A. deKemp, T.D. Ruddy, “Optimization of SPECT Measurement of Myocardial Blood Flow with Corrections for Attenuation, Motion, and Blood-Binding Compared to PET.” J Nucl Med. 2017 Dec;58(12):2013-2019.
- A. Pourmoghaddas, R.G. Wells, “Analytically-Based Photon Scatter Modeling For A Multi-pinhole Cardiac SPECT Camera”. Med Phys 2016 43:6098-6108.
- P.J. Prior, R. Timmins, J. Petryk, J. Strydhorst, Y. Duan, L. Wei, R.G. Wells, “A modified TEW approach to scatter correction for In-111 and Tc-99m dual-isotope small-animal SPECT”. Med Phys 2016 43:5503-5513.
- M. Kamkar, L.Wei, C. Gaudet, M. Bugden, J. Petryk, Y. Duan, H.Wyatt, R.G.Wells, Y. Marcel, N.D. Priest, R. Mitchel, T.D. Ruddy, “Evaluation of Apoptosis with 99mTc-rhAnnexin V-128 and Inflammation with 18FFlurodeoxyglucose in a Low-Dose Irradiation Model of Atherosclerosis in Apolipoprotein E-Deficient Mice.”. J. Nucl. Med. 2016; 57(11):1784-1791.
- J. Wang, R. Arulanandam, R. Wassenaar, T. Falls, J. Petryk, J. Paget, K. Garson, C. Cemeus, B.C. Vanderhyden, R.G. Wells, J.C. Bell, F. Le Boeuf, “Enhancing expression of functional human sodium iodide symporter and somatostatin receptor in recombinant oncolytic vaccinia virus for in vivo imaging of tumors”. J Nucl Med 2017; 58(2):221-227. [Epub 2016 Sep 15].
- H. Gabrani-Juma, O.J. Clarkin, A. Pourmoghaddas, B. Driscoll, R.G. Wells, R.A. deKemp, R. Klein, “Validation of a Multimodality Flow Phantom and its Application for Assessment of Dynamic SPECT and PET Technologies”. IEEE Trans Med Imaging, 2017; 36:132-141
Staff
- Glenn Wells, PhD
Principle Investigator - Sarah Cuddy-Walsh
Postdoctoral Fellow - Dylan Malenfant
PhD student - Taylon Clark
MSc student
Focus
One aspect of our research is the evaluation of new developments in SPECT imaging in a working clinic. This evaluation may involve new imaging equipment, new radio-tracers--the drugs we use to take our pictures--and new image processing techniques. Two examples of recent activities on this front are: evaluation of a new reconstruction algorithm and comparison of two different tracers for measuring blood flow in the heart. Our research projects reflect these different interests.
Projects
CZT Dedicated Cardiac Camera
The new camera design greatly increases sensitivity, giving us more signal – which translates into clearer pictures – while reducing the scan times from 15 minutes to 3 minutes or less.
Development of SPECT Quantitative Myocardial Blood Flow
The design of the new CZT camera allows us to take pictures more rapidly and allows us to measure absolute blood flow in the heart muscle. By taking many pictures of the patient as the tracer is injected, we can see where and how fast the tracer distributes in the body. By analyzing these pictures, we can calculate exactly how much blood flow there is. This is helpful in identifying certain types of heart disease. We are developing this technology here at the Heart Institute and, if successful, it may increase the amount of information available from our SPECT tests. Concurrent with clinical studies (below), we are investigating methods of automated motion correction and advanced reconstruction methods to improve the repeatability (precision) of MBF measurements. This research uses computer simulations and evaluation with both phantom data and clinical patient datasets.
Clinical Evaluation of SPECT Myocardial Blood Flow
We are evaluating and developing SPECT MBF for routine use in the clinic. We have validated the feasibility of measuring flow using large animal models and have demonstrated the accuracy of SPECT MBF measurements in head-to-head studies in patients against the clinical standard of PET MBF imaging. We are also evaluating the repeatability of this technique and leading a multi-center trial to assess the impact of MBF on clinical workflow.
Variable resolution and sensitivity in pinhole SPECT
The new cardiac SPECT camera design uses pinhole collimation which introduces substantial variation into the sensitivity and spatial resolution of the camera over its field of view. We are studying the impact of these variations on the detection of heart disease and investigating camera design modifications that might reduce the effects of these variations. The next stage of this project is to explore the impact of variation in spatial resolution. This work is done using computer modeling and simulation of the pinhole SPECT system followed by experimental validation with the clinical camera.
Scatter modeling
The new cardiac cameras use solid-state Cadmium Zinc Telluride detectors which have superior energy resolution compared to traditional crystal detectors but which also have an energy spectrum that complicates standard methods of correcting for scattered photons. Accurate modeling of scatter for this system may allow improved scatter correction in cardiac imaging and may also offer a means to use the scatter photons for image reconstruction. This latter approach could allow improved image quality and/or reduced radiation dose to patients undergoing these important clinical procedures. With this goal in mind, we are developing and implementing accurate methods of scatter simulation and developing novel reconstruction algorithms that integrate information across multiple energy windows.
Performance Assessment
The novel camera design makes it difficult to assess the performance of these cameras. We are investigating practical methods of measuring camera performance and thereby ensuring that the image quality is maintained at optimal design levels. This project uses experimental measurements and comparisons of system performance for several cardiac SPECT systems. Our goal is to develop quality assurance tests and procedures that will ensure optimal performance.
Small Animal Studies
Studying disease in small animals is very helpful for understanding the causes of heart disease, for creating better ways to detect disease, and for developing new ways of treating disease. To maximize the value of these studies and to develop new understanding as quickly as possible, we use the same tools in animals as we do for people. At the Heart Institute, we have the capability to take SPECT and CT images of small animals and are doing so to develop new ways of imaging disease.
Gallery
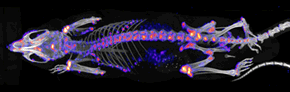
A mouse MDP (methylene diphosphonate) bone metabolism SPECT/CT study. This is used in nuclear medicine for several indications including the spread of cancer to the bones. The CT is shown in gray-scale while the SPECT bone metabolism data is shown in pseudo-colour where red/white indicates high metabolism and blue/black indicates low. There is no disease in this image.
A beating heart image of blood flow in a rat heart (300 grams). We use ECG gated studies to assess the function of the heart and measure parameters like ejection fraction, heart volume, and to assess the quality of blood flow to heart tissues. Four views (top left to bottom right): a maximum-intensity reprojection, followed by sagital, coronal, and transverse views. This is a healthy heart.
The same configuration for a mouse (30 grams).
An image of cell death in vivo. The red shows blood flow image in a rat that has had a large heart attack. The green shows dying cells. Note the expression of cell death in the area of heart without blood flow. (There is also uptake in extra-cardiac structures associated with the disease model.)


